As humanity becomes smarter everyday, our societies and wold progress. Advances in technology have resulted in computers at our fingertips, environmentally friendly cars, and even footage with audio straight from Mars! Innovation drives progress, and this also applies to healthcare. Everyday scientists are researching to design and create more efficient and ideal drugs to treat every type of medical condition and disease.
The FDA’s Center for Drug Evaluation and Research (CDER) works along side the scientists and pharmaceutical companies to annually approve a wide range of new drugs and biological products.
Listed are a few of the newly “FDA- approved use”:
| No. | Drug Name | Active Ingredient | Approval Date | FDA-approved use on approval date* |
| 15 | Qelbree | viloxazine | 4/2/2021 | For the treatment of attention deficit hyperactivity disorder |
| 14 | Zegalogue | dasiglucagon | 3/22/2021 | To treat severe hypoglycemia |
| 13 | Ponvory | ponesimod | 3/18/2021 | To treat patients with relapsing forms of multiple sclerosis |
| 12 | Fotivda | tivozanib | 3/10/2021 | To treat patients with renal cell carcinoma |
| 11 | Azstarys | serdexmethylphenidate and dexmethylphenidate | 3/2/2021 | For the treatment of Attention Deficit Hyperactivity |
| 10 | Pepaxto | melphalan flufenamide | 2/26/2021 | For the treatment of certain patients with relapsed or refractory multiple myeloma |
| 9 | Nulibry | fosdenopterin | 2/26/2021 | To treat patients with the rare genetic disease molybdenum cofactor deficiency Type A |
| 8 | Amondys 45 | casimersen | 2/25/2021 | For the treatment of Duchenne muscular dystrophy |
| 7 | Cosela | trilacicilib | 2/12/2021 | To mitigate chemotherapy-induced myelosuppression in adult patients with small cell lung cancer |
| 6 | Evkeeza | evinacumab-dgnb | 2/11/2021 | For the treatment of homozygous familial hypercholesterolemia |
| 5 | Ukoniq | umbralisib | 2/5/2021 | For the treatment of certain patients with marginal zone lymphoma and follicular lymphoma |
| 4 | Tepmetko | tepotinib | 2/3/2021 | To treat non-small cell lung cancer |
| 3 | Lupkynis | voclosporin | 1/22/2021 | To treat lupus nephritis Drug Trials Snapshot |
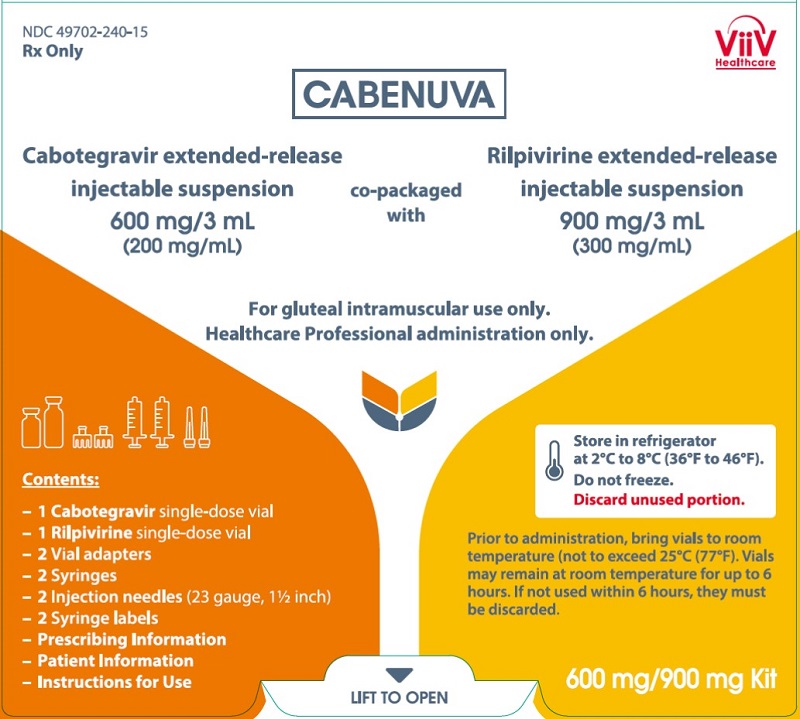
| 2 | Cabenuva | cabotegravir and rilpivirine | 1/21/2021 | To treat HIV Drug Trials Snapshot |
| 1 | Verquvo | vericiguat | 1/19/2021 | To treat chronic heart failure Drug Trials Snapshot |
(1)
A noteworthy novel drug is Cabenuva. This is the first extended-release, injectable drug regimen for adults with HIV. It has been approved as a complete regimen for the treatment of HIV-1. This is the first FDA approved injectable, complete regimen for adults that is administered once a month. The current standard of care for patients with HIV is to take pills daily to manage conditions. This gives patients the option to have one monthly injection instead of daily oral regimen.
As new treatment options and medications make their way from the laboratory to the patient, most of them find their way through the pharmacy. The pharmacist and pharmacy technician play a large and vital role in delivering medications to patients. In many instances the physician will consult the pharmacist to ensure that they prescribe the correct medication. The pharmacist has a strong understanding of pharmacology and will take each patient’s pre-existing conditions and of medications into consideration when recommending the ideal prescription.
A great way to enter into the field of pharmacy is through HealthCareer Certs. They offer a 12- week online, module based course. Upon completion of the course you will take the national exam to obtain a nationally accredited certification as a pharmacy technician (CPhT). Becoming a CPhT will open doors to not only a great career, but it is also a great opportunity as a pre-healthcare career. The experience of working in the healthcare field, one on one with a pharmacist is a one of a kind opportunity. Not only is it a great career, it will make you a more competitive applicant for either, pharmacy school, medical school, dental school, and more!
References
1. Novel Drug Approvals for 2021. https://www.fda.gov/drugs/new-drugs-fda-cders-new-molecular-entities-and-new-therapeutic-biological-products/novel-drug-approvals-2021.